 Accel Research Sites named top enroller for cutting-edge Alzheimer’s disease clinical trial
Accel Research Sites named top enroller for cutting-edge Alzheimer’s disease clinical trial

Create a Profile

Create a Profile

Get In Touch Mobile
COVID-19 Vaccines for Children: What We Saw in Clinical Trials
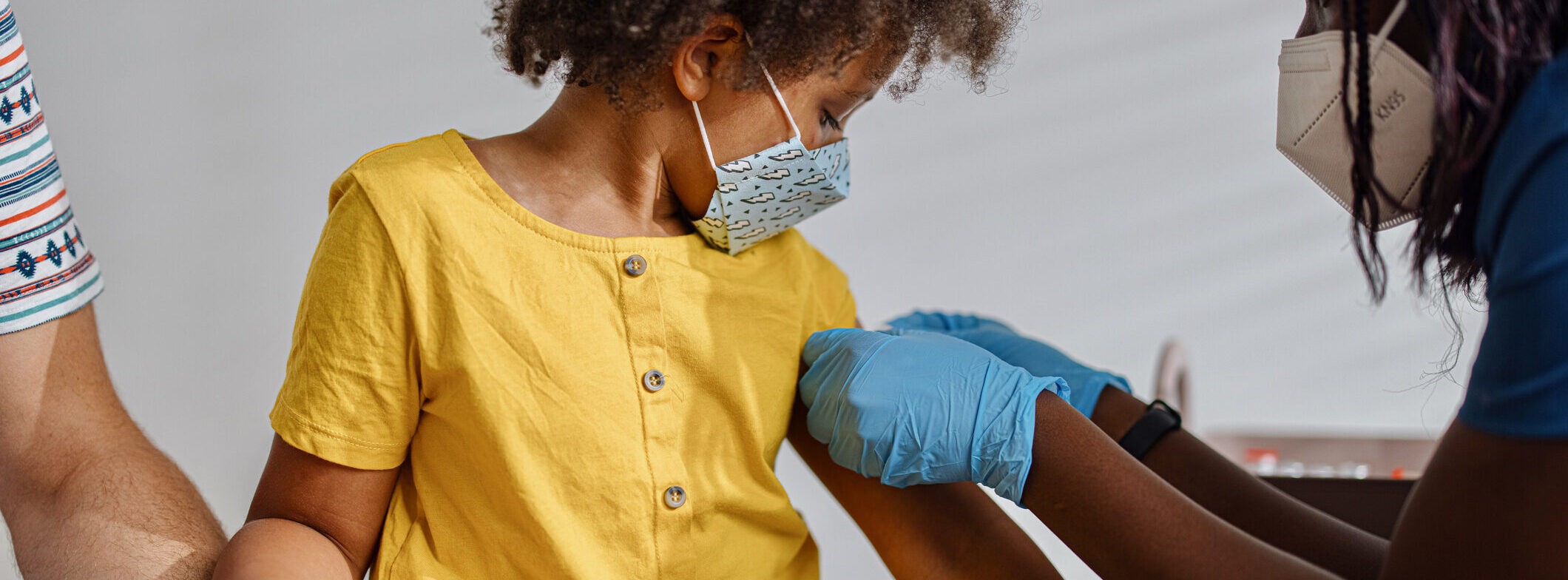
Early in November 2021 the CDC recommended that children ages 5 to 11 be vaccinated against COVID-19 with the Pfizer vaccine.
Even so, it’s understandable that some parents might have questions and concerns about the vaccine. They might worry about how fast the vaccines were developed and approved, or wonder why children should get the COVID-19 vaccine if they don’t experience severe illness as frequently.
At Accel Research Sites, we have been working through our extensive network of physicians to study the COVID-19 vaccine candidates from the very beginning. That includes pediatric COVID-19 vaccines. Through this work and their vast experience, our physicians have a unique, first-hand perspective on these vaccines.
Dr. Salma Elfaki is one of those physicians. She owns Nona Pediatric Center in Orlando and was a principal investigator on the Moderna COVID-19 teen vaccine clinical trial. She shared some of her observations of the clinical trial process and her insights on why parents should get their children vaccinated.
How the pediatric COVID-19 vaccine clinical trials were conducted
Dr. Elfaki noted that 2,268 children were enrolled in the Pfizer pediatric clinical trial. 1,517 received a vaccine dose, and 751 received a placebo. The study, she noted, was a double blind, randomized, placebo-controlled study, meaning neither the participant nor the researcher knew who had been administered the vaccine vs. placebo. This is the gold standard in research, she said.
Children were given two doses equal to 1/3 of the adult dosage of the Pfizer vaccine, administered three weeks apart, and were followed for at least two months after the second dose.
What we saw from pediatric COVID-19 vaccine clinical trials
The vaccine was found to have a 90.7 percent efficacy rate in preventing COVID-19 infection, with just three vaccinated participants contracting the disease with mild infections vs. 16 unvaccinated participants. Children also produced excellent levels of antibodies with the smaller dose.
Additionally, there were no serious complications of myocarditis or MIS-C (multi-system inflammatory syndrome). The most common side effects were injection site pain, fatigue, headache, body aches and chills within 48 hours of injection. These resolved quickly.
What should parents do regarding the pediatric COVID-19 vaccine?
Even with the encouraging data, parents might be wondering if they should vaccinate their children against COVID-19.
Dr. Elfaki said that though people might think children in this age group won’t get sick or won’t get sick as severely, 1.9 million children ages 5 to 11 have been infected, accounting for 9 percent of U.S. cases. This has resulted in 8,300 hospitalizations and 94 deaths, making COVID-19 the eighth leading cause of death for children in this age group.
Vaccination, she said, can reduce their risk of contracting the disease by 90 percent, helping to protect them again hospitalization, long haul COVID or even death.
But worst-case scenarios aside, vaccination still has big benefits, Dr. Elfaki noted. With vaccinated children, parents don’t have to worry and test at every small symptom. It can also help keep kids in school and eventually, keep them in school without masks. And, on the largest scale, vaccinations for children 5-11 help prevent them from spreading the virus and help all of us end the pandemic.
We believe in the research Dr. Elfaki and thousands of pediatricians across the country have conducted on pediatric vaccines. We’re incredibly proud to have been part of the solution, helping to move medicine forward and end this pandemic.
If you’re a parent of a child eligible for the vaccine, we encourage you to speak with your primary care physician. They can help answer your questions and make the best decision for you and your child.
Articles
 Accel Research Sites named top enroller for cutting-edge Alzheimer’s disease clinical trial
Accel Research Sites named top enroller for cutting-edge Alzheimer’s disease clinical trial
 Principal investigator discusses increase in overdoses associated with fentanyl
Principal investigator discusses increase in overdoses associated with fentanyl
 Tampa TV station shares stories of participants in Alzheimer’s trial
Tampa TV station shares stories of participants in Alzheimer’s trial
Ready to be part of healthcare history? Find the right clinical trial for you.


